Testing Solutions that Accelerate Development and Manufacturing of mRNA- and Protein-based Vaccines
InDevR offers both off-the-shelf and custom analytical solutions designed to expedite the development, optimization, and production of multiple types of vaccines, including mRNA, polysaccharide, conjugate, and viral vector vaccines.
The VaxArray Platform and multiplex assays are built to deliver rapid results and reliable data, ensuring customers meet the high-quality standards needed to navigate the global regulatory environment.
Improve Productivity Using Microarray Technology
Enabling multiple assays in a single multiplex array, the VaxArray Platform eliminates extra process steps, providing faster turnaround times compared to ELISA, flow cytometry, HPLC, and Luminex workflows with automated results in under 2 hours.
Adhere to Global Regulatory Compliance and GMP
21 CFR Part 11 compliant enabling software ensures data integrity and security for regulatory requirements, making us globally recognized by international regulatory agencies for testing in a GMP environment and product release.
Streamline Vaccine Development
Analyze various sample types, including antigens, antibodies, and oligos and states from crude matrices to formulation across all stages of vaccine development, including R&D, clinical trials, fit-for-purpose, and release testing.
Support Global Quality Initiatives
Utilizing standardized methods and technology on a user-friendly benchtop platform improves user-to-user and site-to-site standardization, simplifies operations, reduces the likelihood of user error, and meets global quality goals.
It’s in the Tech–Versatile Multiplex Immunoassays with Unlimited Potential
Explore the possibilities with immunoassay arrays that deliver almost any configuration of antibody, antigen or oligo to meet your specific vaccine testing needs. InDevR’s core technology for flexible microarray slide printing offers up to 24 wells per slide and over potential 50 assays in each well. In a single 1-hour run, you can simultaneously run 4 microarray slides, capturing over 5,000 analyses–saving you time and resources.
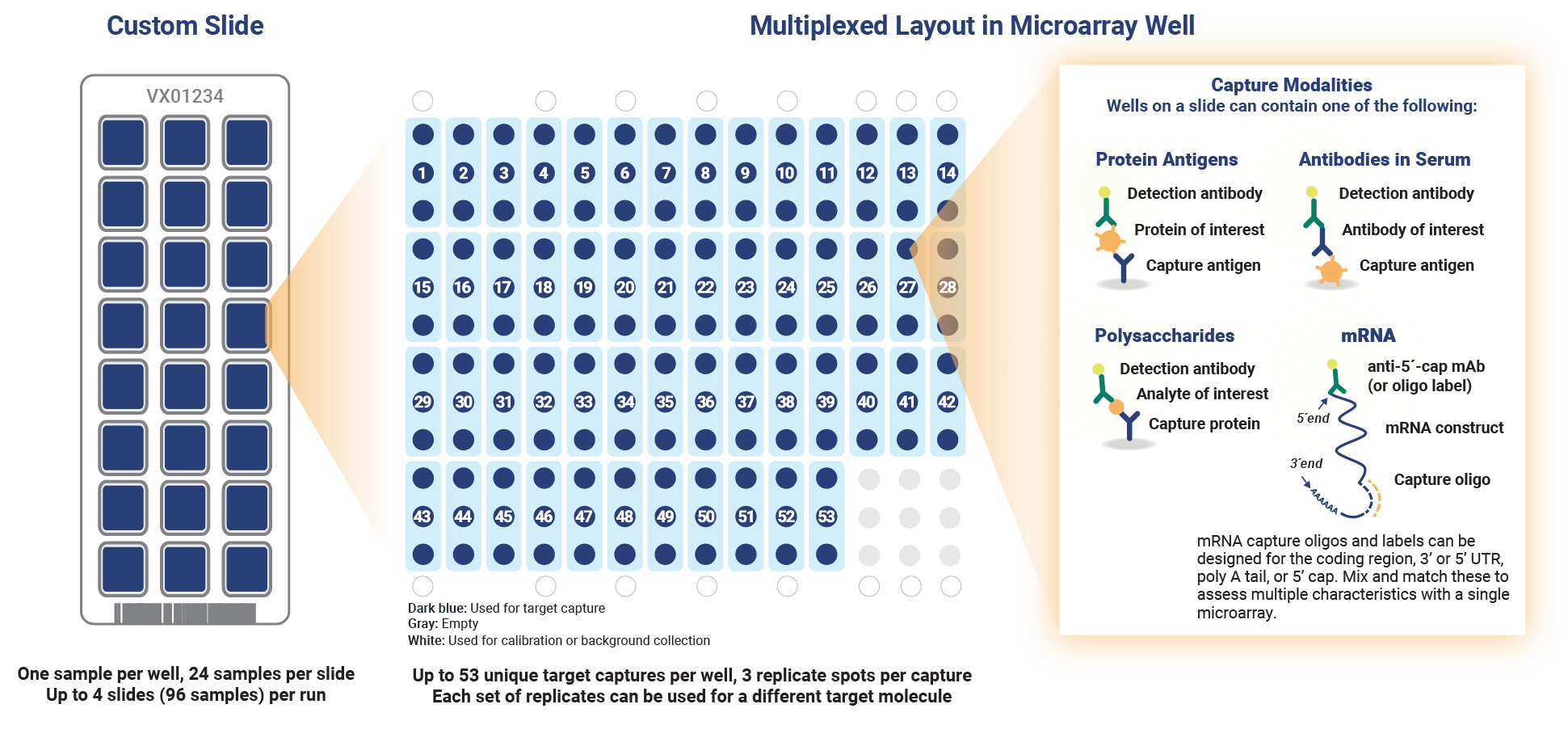
Custom Solutions for Your Novel Challenges
Let InDevR design a customized microarray assay that accelerates your development and manufacturing bioprocesses. With over two decades of experience in custom vaccine assay development, our Expert Services Team can help you address and eliminate bottlenecks, expedite your vaccine analytics, and save months of developing, optimizing and validating assays in-house.
Let our team develop assays to help you detect and quantify mixtures of multiple proteins, from vaccine antigens to cellular expression of proteins following mRNA transfection.
Our team can design assays to quantify multivalent vaccine-induced antibody response levels in serum that meet your needs.
We can create assays to detect and quantify highly valent mixtures of pneumococcal polysaccharides and conjugate antigens.
We develop custom assays to measure the identity and quantity of capped and intact mRNA constructs in multivalent formulations.
Custom Solutions for Your Novel Challenges
Let InDevR design a customized assay that accelerates your development and manufacturing bioprocesses. With over two decades of experience in custom vaccine assay development, our Expert Services Team can help you address and eliminate bottlenecks, expedite your vaccine analytics, and save months of developing, optimizing and validating assays in-house.
Eliminate Months of Seasonal Influenza Assay Development
Ready-to-go assays for seasonal WHO strains
Every flu season, the WHO evaluates new influenza strains for potential vaccine inclusion. Their recommendations often require updated analytical methods, increasing the test development burden on vaccine developers and manufacturers. InDevR does all of this development, optimization, and validation work up front, offering ready-to-go, pre-validated VaxArray influenza kits every season.
InDevR’s commitment to ensuring that the reagent kits are responsive to seasonal influenza strain changes saved our development team significant time and resources.”
-Michael Schrader, Co-founder of Vaxess Technologies