Rapid Multiplexed Solutions for mRNA Applications
Eliminate mRNA analytical development bottlenecks and accelerate your path to market with custom multiplex mRNA solutions.
InDevR is your trusted partner for multiplexed assay development and integration during production and release of high-valency mRNA drug products, confirmation of protein expression, and serological testing in clinical and pre-clinical trials.
mRNA Assay Capabilities
Custom Analytical mRNA Solutions
Confirm identity and measure quantity of multivalent naked or LNP-encapsulated mRNA in < 2 hours with our flexible platform.
Measure Intact mRNA and 5’ Capping in 1 assay
Rapidly measure capped and intact mRNA and 5’ capping efficiency at your benchtop. InDevR’s off-the-shelf 5’CapQ Assay measures mRNA intactness with an easy-to-use microarray-based platform.
Universal Influenza HA mRNA Identity and Quantity
Quantify multivalent influenza HA mRNA regardless of different codon optimization schemes. mRNA fluIQ is designed to provide universal reactivity with HA mRNAs.
Multiplexed Influenza Protein Quantification
InDevR offers off-the-shelf VaxArray Assays that target hemagglutinin (HA), neuraminidase (NA) and nucleoprotein (NP) for multiplexed protein quantification from mRNA-transfected cell culture.
Custom Protein Quantification Assay forCustomer-specific Antigens
If one of our off-the-shelf-kits doesn’t meet your needs, our Expert Services Team can design a custom assay for you. An immunoassay specific to the target antigen can be developed for rapid and accurate quantification post-transfection.
Analyze Serum Samples Against Your Antigens
Accelerate pre-clinical and clinical trial testing by having our Expert Services team develop an assay to rapidly quantify the antibody response to specific antigens in your serological samples. VaxArray multiplexing capabilities enable evaluation of immunogenicity for all antigen targets in your multivalent mRNA drug product in a single test, increasing sample throughput and analysis during drug development.
Simplify mRNA Analytics
mRNA integrity and identity mean different things to different people. Work with InDevR’s Expert Services Team to design custom-tailored solutions for your mRNA sequences utilizing the flexibility of the VaxArray Platform. Custom assays can be developed for quantity, identity, integrity, 5’ capping efficiency, and 3’-polyA tailing efficiency, and are compatible with pre- and post-LNP encapsulated samples without additional processing steps.
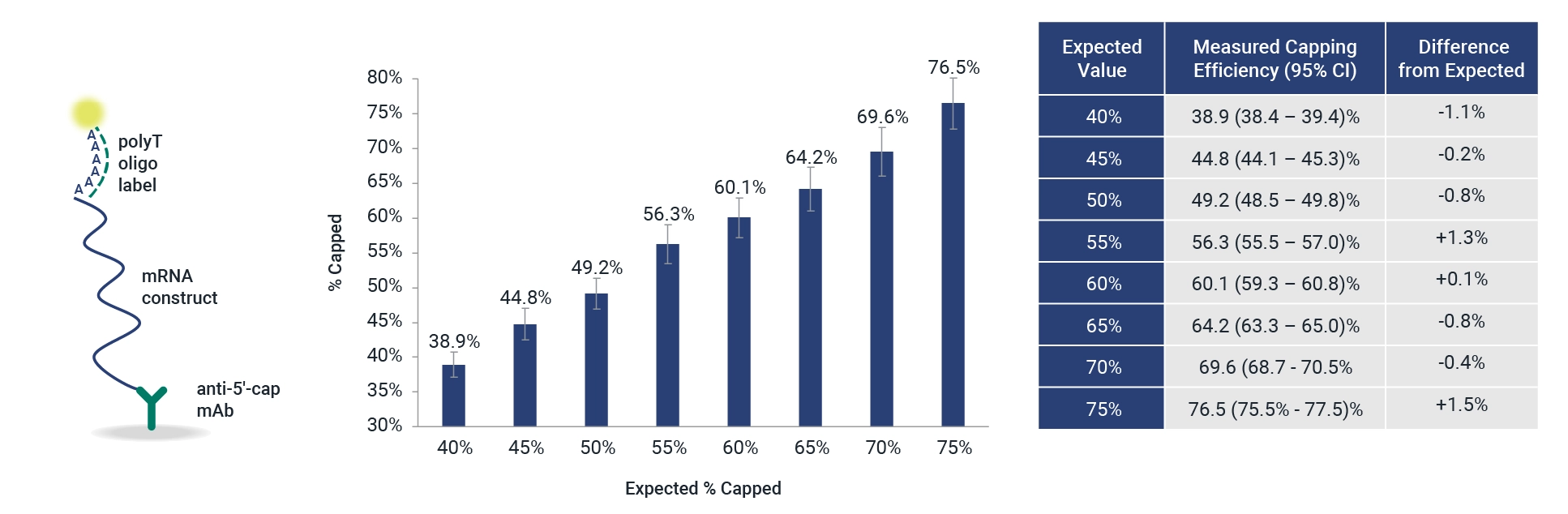
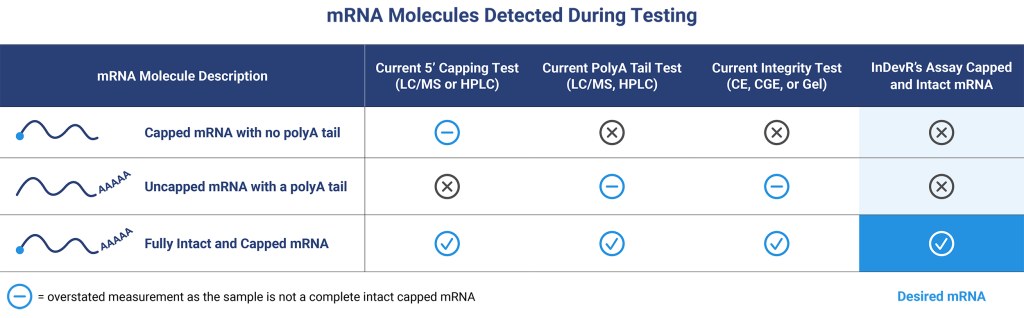
Rapid Measurement of 5′ Capping Efficiency and Intact mRNA Testing
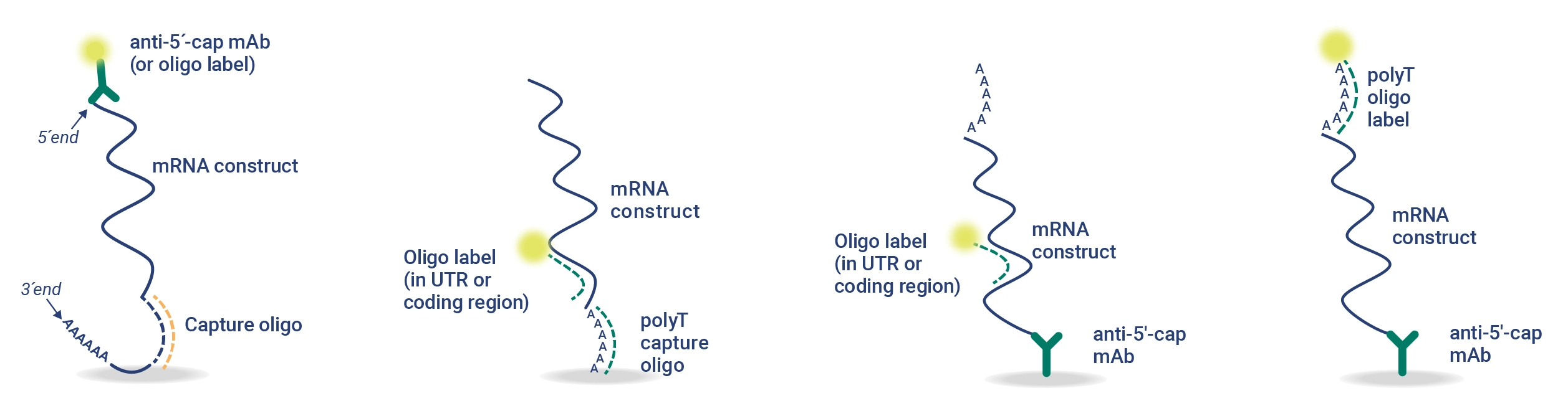
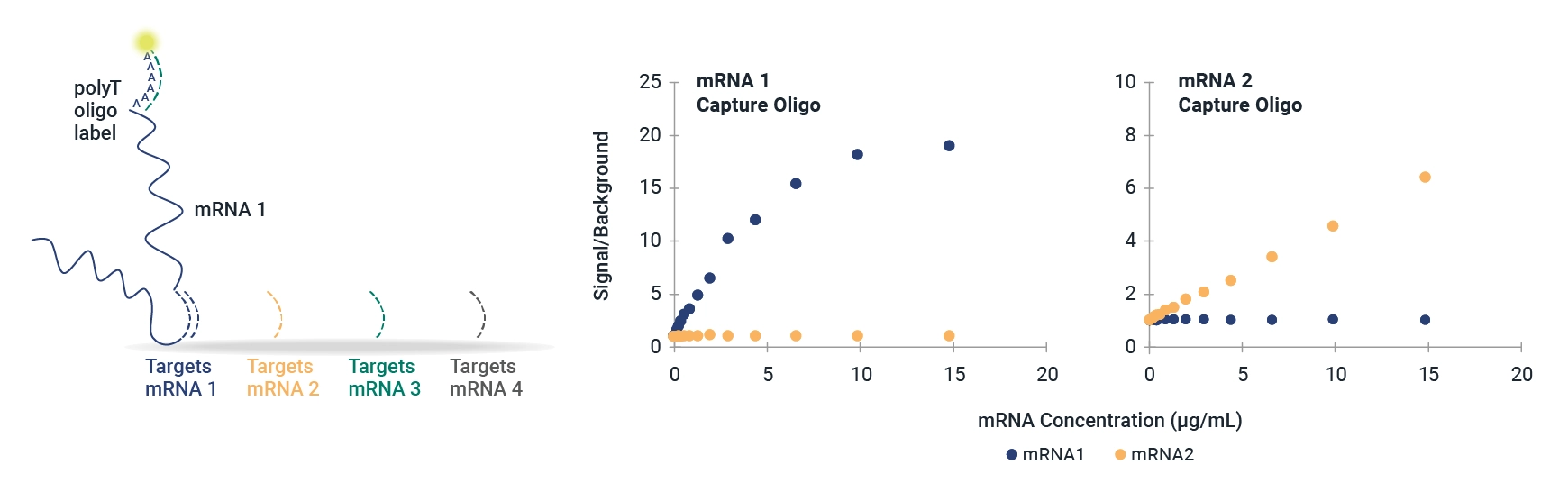
The 5′ cap and intact poly(A) tail structures of mRNA are important for protein expression and mRNA stability. Current vaccine companies use complex chromatographic methods that can take 1-2 days with additional queue and analysis time to measure mRNA concentration, intactness, and purity. The 5’CapQ Assay is a simple measurement of capped and intact mRNA that can be completed in < 2 hours on a user-friendly system that doesn’t require an expert to operate. This assay works by capturing the mRNA molecules via the 5’ cap and labeling the molecules on the poly(A) tail, enabling quantification of only the complete intact mRNA molecules. RNA molecules that lack a 5’ cap or poly(A) tail are not measured in the 5’CapQ Assay, leading to a single test for quantifying your mRNA.
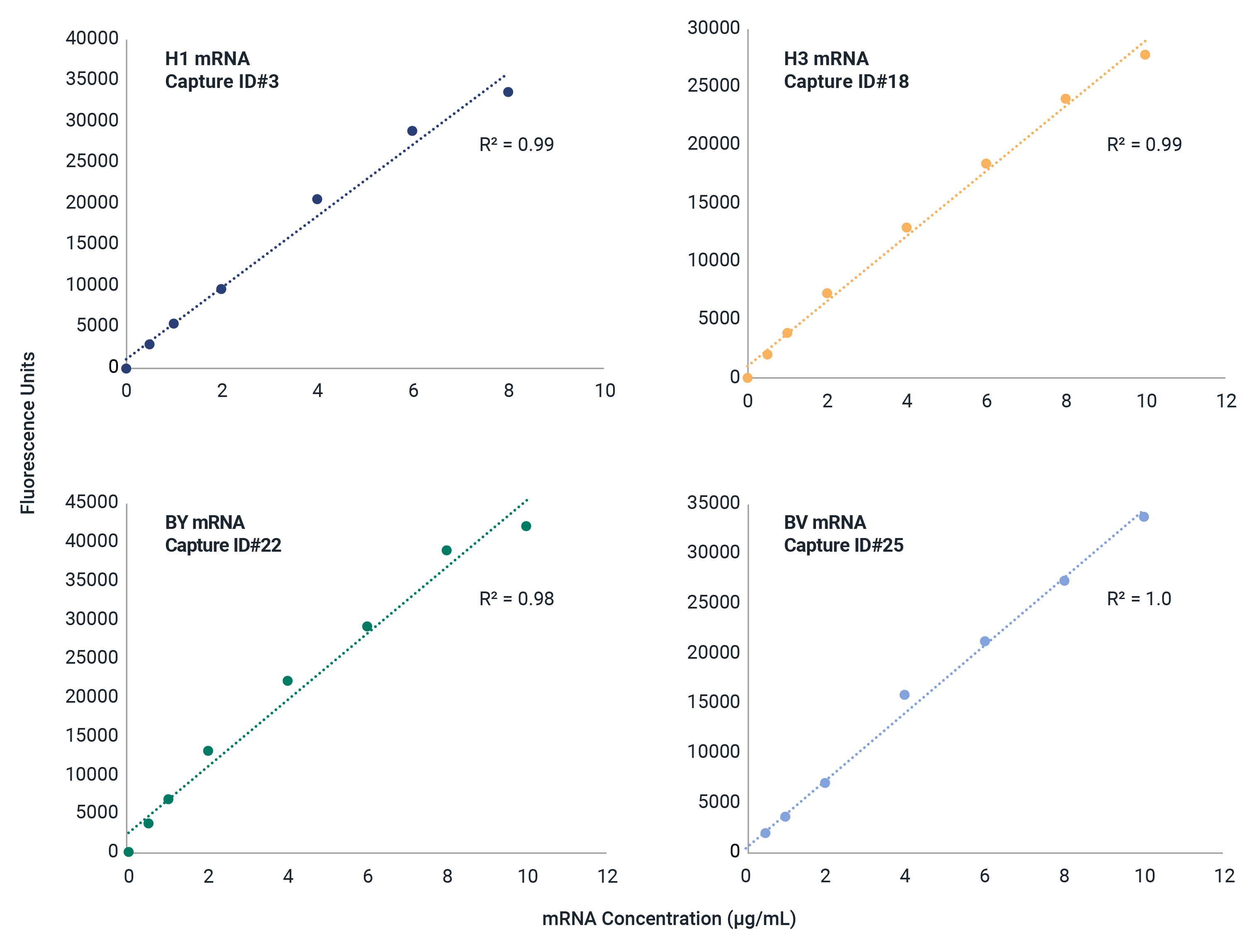
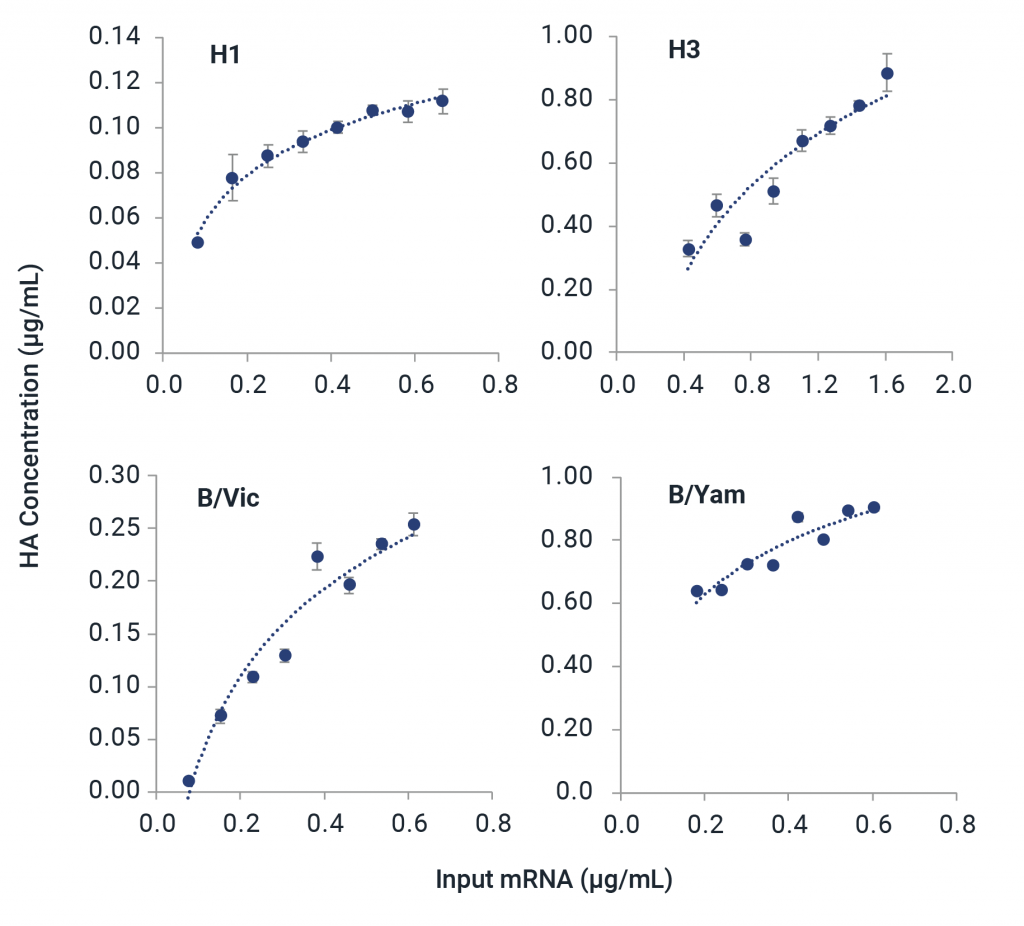
Universal Influenza HA mRNA Identity and Quantity
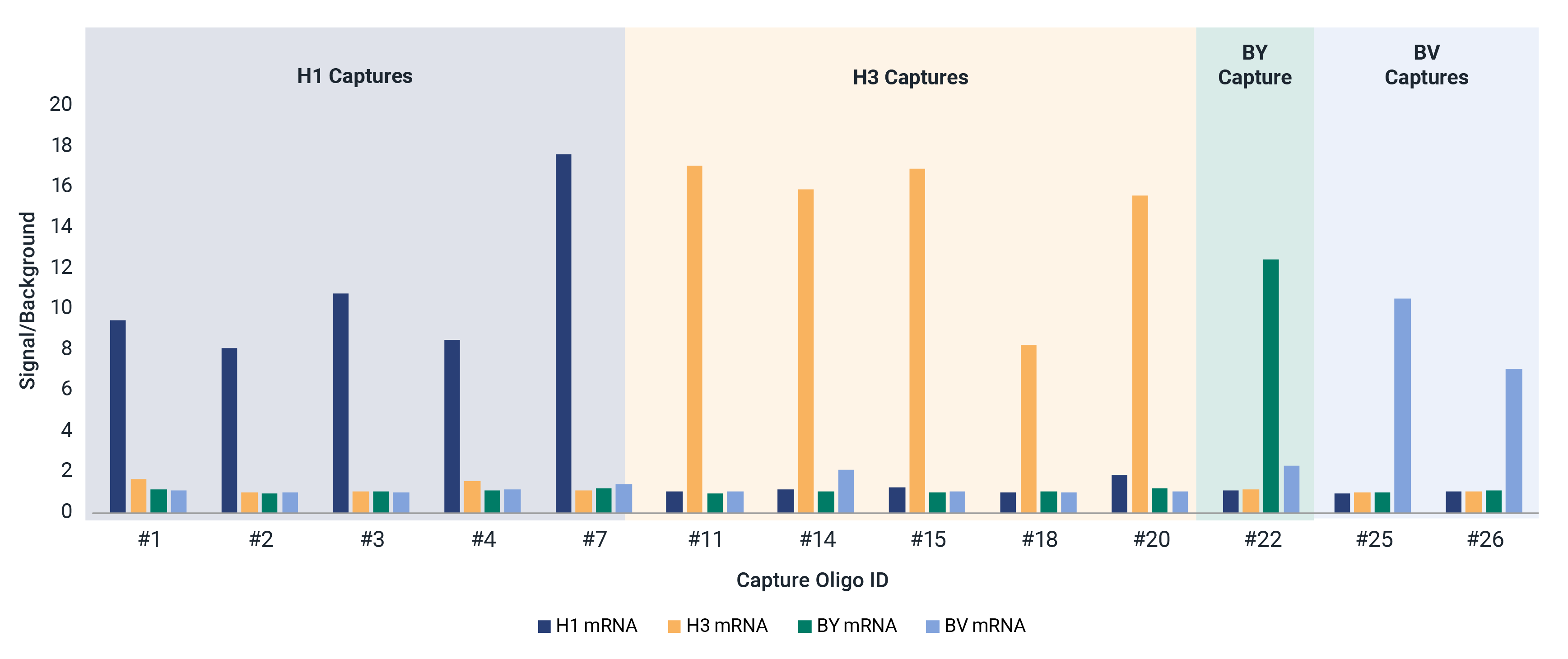
The mRNA fluIQ Assay contains multiple capture oligos specific for influenza A/H1, A/H3, B/Yamagata, and B/Victoria hemagglutinin (HA) vaccine components, and has been strategically designed for different codon optimization schemes to provide universal reactivity with HA mRNAs.
- Reactive to naked and LNP-encapsulated mRNA without a separate extraction step.
- Demonstrated compatibility with strains over 11 vaccine seasons with different codon optimization schemes and UTRs.
InDevR’s Expert Services team can perform sample testing with your specific mRNA constructs. Contact us to send your influenza HA mRNA constructs for testing.
Simultaneous Multivalent Protein Characterization
InDevR offers off-the-shelf VaxArray Assays for influenza HA, NA, and NP characterization after mRNA transfection in cell culture.
- Assess multivalent expressed proteins with high specificity for mRNA construct development and optimization, and product release.
- HA and NA assays with vaccine component specificity enable simultaneous quantification of expressed protein for multivalent mRNA formulations. Reactivity is re-evaluated semi-annually with updated vaccine strain recommendations.
- Stability-indicating immunoassay measures biologically-relevant forms of expressed HA and NA.
Evaluate functional protein during optimization of transfection conditions. Contact us for more information on our ready to go kits.
Publications
Gao R.Y., et al. Rapid identity and quantity CQA test for multivalent mRNA drug product formulations. 2022. Vaccines. 10(10): 1704. doi: 10.3390/ vaccines10101704
Gerold, M. et al. Analytical Performance of a Multiplexed Microarray Assay for Rapid Identity and Quantification of a Multivalent mRNA Vaccine. 2024. SSRN. http://dx.doi.org/10.2139/ssrn.4849255