Accelerate Your Vaccine Development and Manufacturing
The VaxArray® Platform is now compatible with 96-well plate format. This next-generation vaccine testing solution now provides automation-friendly solutions that can be integrated into your automated workflow.
The VaxArray Difference for Vaccine Analytics
VaxArray products provide solutions for vaccine manufacturers at multiple stages: from challenges in early development and optimization, to pre-clinical and clinical evaluation, to release testing including identity and potency — all with a single platform. Multiplexed immunoassays provide unparalleled efficiency and precision to improve at-line analysis, and empower researchers to obtain faster, more reliable results in their efforts to advance public health.

 VaxArray products provide solutions for vaccine manufacturers at multiple stages: from challenges in early development and optimization, to pre-clinical and clinical evaluation, to release testing including identity and potency — all with a single platform. Multiplexed immunoassays provide unparalleled efficiency and precision to improve at-line analysis, and empower researchers to obtain faster, more reliable results in their efforts to advance public health.
VaxArray products provide solutions for vaccine manufacturers at multiple stages: from challenges in early development and optimization, to pre-clinical and clinical evaluation, to release testing including identity and potency — all with a single platform. Multiplexed immunoassays provide unparalleled efficiency and precision to improve at-line analysis, and empower researchers to obtain faster, more reliable results in their efforts to advance public health.
Measure Antigens, Antibodies or Nucleic Acids with One Platform
The VaxArray Platform integrates multiplex capability onto a microarray slide that can be used to measure antigen content, protein expression, serological changes in antibody response, and multiplex mRNA constructs.
Streamlined Path to Validated Assays
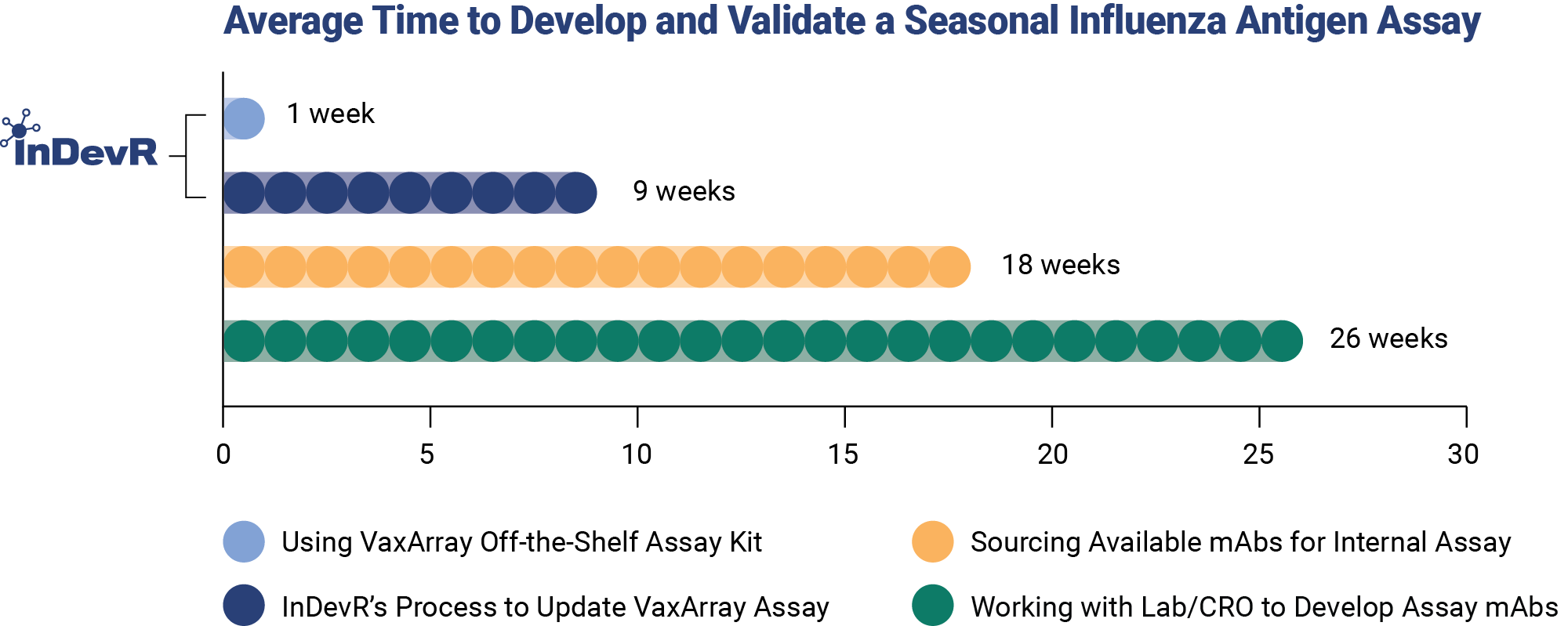
Simplify workflows by validating your analytical methods in weeks instead of months.
Seamless Integration
VaxArray integrates with existing workflows, maintaining your current 96-well format, allowing for an efficient transition and sustained productivity.
Simple-to-Use
Standardize your antigen and serology testing with rapid, ready-to-go VaxArray kits.
Multiplex 50+ Analytes in Triplicate
VaxArray multiplexed technology outpaces traditional single-plex methods like ELISA in speed and efficiency, while improving statistical robustness of your data.
Benchtop Microarray Assays
Compact, user-friendly platform brings high-throughput multiplex capabilities right to your benchtop.
Versatile Platform
Analyze a broad spectrum of vaccine-relevant samples, from antibodies and antigens to nucleic acids all on one platform.
| Streamlined Path to Validated Assays
Simplify workflows by validating your analytical methods in weeks instead of months. |
|
| Seamless Integration
VaxArray integrates with existing workflows, maintaining your current 96-well format, allowing for an efficient transition and sustained productivity. |
|
| Simple-to-Use
Standardize your antigen and serology testing with rapid, ready-to-go VaxArray kits. |
|
| Multiplex 50+ Analytes in Triplicate
VaxArray multiplexed technology outpaces traditional single-plex methods like ELISA in speed and efficiency, while improving statistical robustness of your data. |
|
| Benchtop Microarray Assays Compact, user-friendly platform brings high-throughput multiplex capabilities right to your benchtop. |
|
| Versatile Platform
Analyze a broad spectrum of vaccine-relevant samples, from antibodies and antigens to nucleic acids all on one platform. |
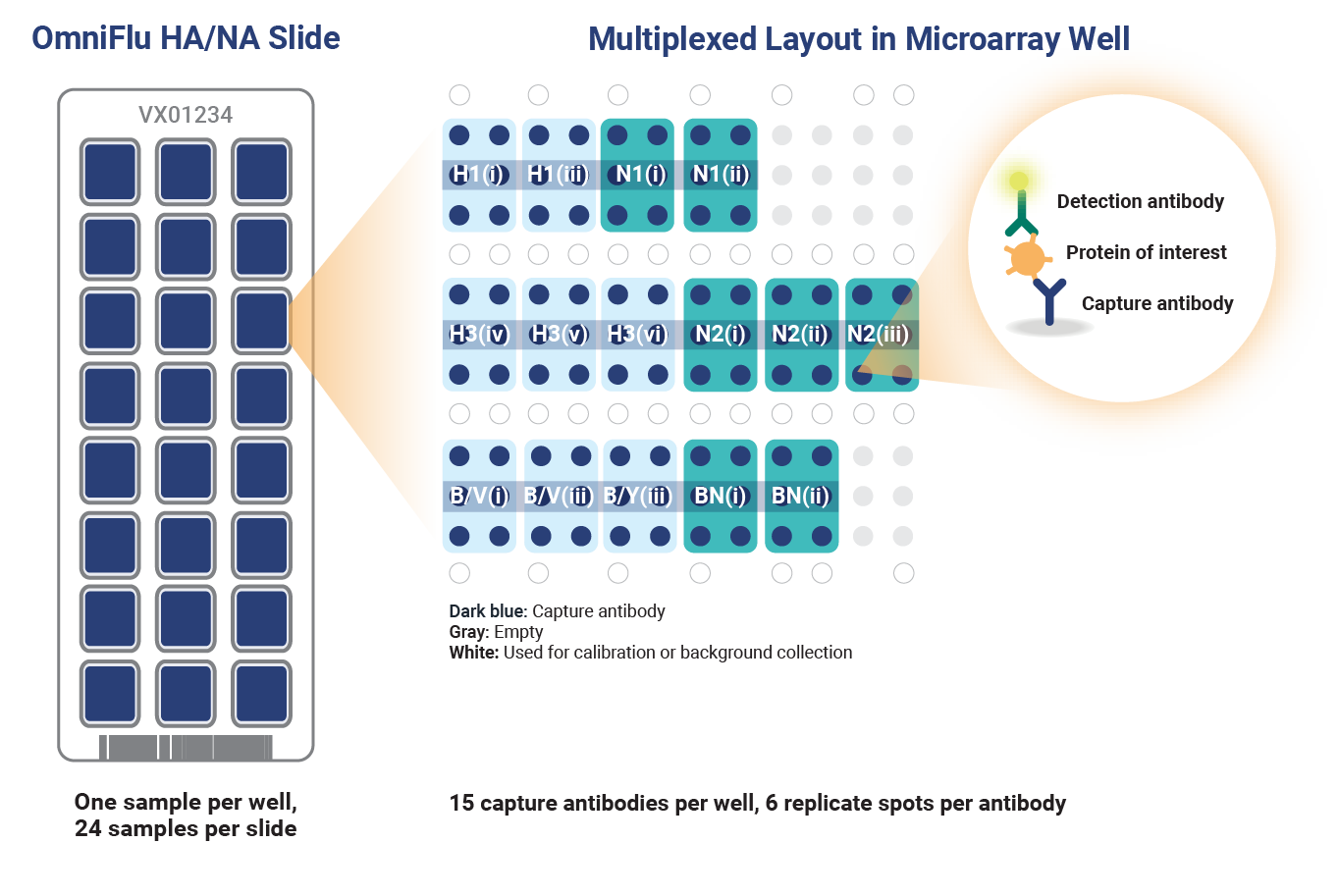
OmniFlu HA/NA 96 microarray slide layout. OmniFlu HA/NA 96 targets 7 unique proteins (influenza HA from H1, H3, B/Victoria and B/Yamagata, and influenza neuraminidase subtypes N1, N2, and B/NA) but contains multiple antibodies for each seasonal influenza subtype and lineage (except for B/Yamagata). This better ensures reactivity of at least one capture antibody per subtype or lineage depending on production method and strain.
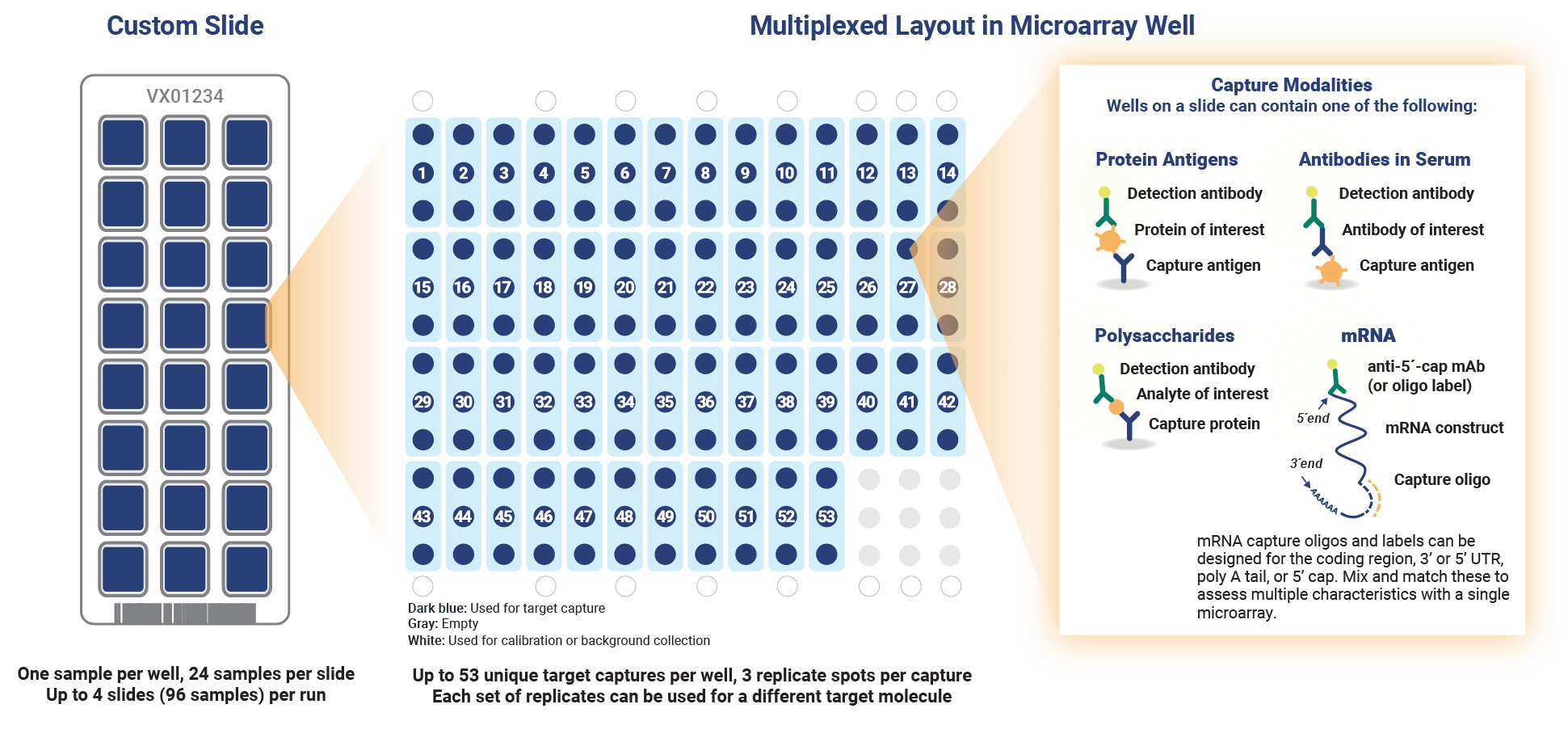
Custom microarray slide layout. Custom assays can be developed for specific antibodies, antigens, polysaccharides or nucleic acids for up to 53 unique targets consolidated into a single test.
Maximize Efficiency
VaxArray Assays outperform traditional ELISAs and other multiplexed immunoassay platforms in critical areas of vaccine testing. Designed with a focus on the vaccine production cycle, the VaxArray Platform facilitates standardized testing, ensuring consistency and reliability from start to finish.
| ELISA | Plate-Based Immunoassays | Bead-Based Immunoassays | VaxArray | |
|---|---|---|---|---|
| Time to Result | 8–24 hours | 24 hours | 24 hours | 1–4 hours |
| Multiplexed | ✗ | ✓ | ✓ | ✓ |
| Seasonally Updated Reagents | ✗ | ✗ | ✗ | ✓ |
| Vaccine-Specific Assays | ✗ | ✗ | ✗ | ✓ |
Expert Solutions for Development Challenges
The vaccine development process is complex. InDevR’s expertly curated repository of monoclonal antibodies targeting influenza, the reliability and precision of the VaxArray Platform, and assays customized to meet your specific needs can eliminate months developing, optimizing and validating in-house assays.
Simplify and Standardize Workflows
Validated, Off-the-Shelf Kits
A variety of ready-to-go kits enable vaccine developers and manufacturers to simplify and standardize workflows. Multiplexed capability allows for simultaneous analysis of multiple, unique targets to reduce testing time and kits are validated to work for many different sample types.
Consistent Results Across Facilities
VaxArray Kits are thoroughly evaluated and manufactured under an ISO13485-accredited quality system to meet specific performance criteria. Our rigorous manufacturing process ensures consistency in results and improves standardization across your facilities.
Flexible, Customizable Multiplexing
InDevR’s flexible microarray printing can deliver almost any configuration of antibody, antigen or oligo to meet your specific vaccine testing needs. Customized multiplexing capability enables simultaneous capture of 1-53 unique target molecules per sample well in triplicate.
| Maximum Number of Results | ||||
|---|---|---|---|---|
| VaxArray Assay Kit | Unique Targets* | Qualitative Analysis |
Quantitative Analysis in Singlicate | Quantitative Analysis in Triplicate |
| OmniFlu HA/NA 96 Assay Kit*** | 7 | 672 | 609 | 196 |
| OmniFlu HA Assay Kit** | 4 | 256 | 220 | 68 |
| Influenza Seasonal NA Multivalent Assay Kit** | 3 | 192 | 165 | 51 |
| Influenza Pandemic HA Multivalent Assay Kit** | 3 | 192 | 165 | 51 |
| Influenza NP Multivalent Assay Kit** | 2 | 128 | 110 | 34 |
| Influenza Monovalent Assay Kits** (A/H1, A/H3, B/Yamagata HA, B/Victoria HA) |
1 | 64 | 55 | 17 |
| Pneumococcal Assay Kit** | 24 | 1536 | 1320 | 408 |
| Coronavirus Spike Protein Multivalent Assay Kit** | 1 | 64 | 55 | 17 |
| Coronavirus SeroAssay Multivalent Assay Kit** | 7 | 448 | 385 | 119 |
| Polio Multivalent Assay Kit** | 3 | 192 | 165 | 51 |
| Custom Assay Kits*** | Up to 53 | 5088 | 4611 | 1484 |
*Per sample in one well.
**16 wells per microarray slide, 4 slides (64 wells) per run. Qualitive analysis: number of unique targets for 64 wells. Quantitative analysis in singlicate: number of unique targets for 55 wells, 8 wells for standard curve, 1 well for positive control. Quantitative analysis in triplicate: 17 samples in triplicate, 8 wells for standard curve, 1 well for positive control in triplicate, 2 blank wells.
***24 wells per microarray slide, 4 slides (96 wells) per run. Qualitive analysis: number of unique targets for 96 wells. Quantitative analysis in singlicate: number of unique targets for 87 wells, 8 wells for standard curve, 1 well for positive control. Quantitative analysis in triplicate: 28 samples in triplicate, 8 wells for standard curve, 1 well for positive control in triplicate, 2 blank wells.
VaxArray Platform
Instrument
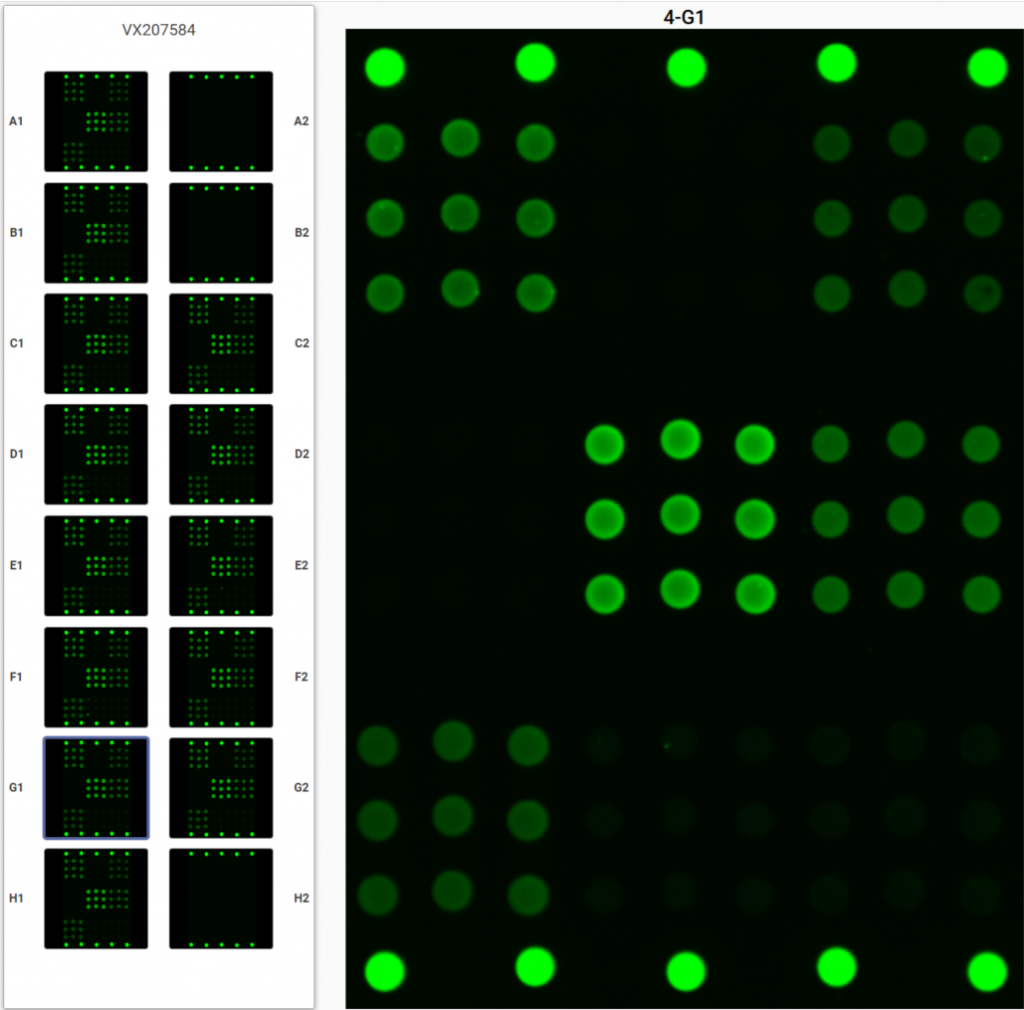
The VaxArray instrument rapidly generates high-resolution fluorescence images of each microarray, analyzes the data and provides test results. Analyze up to 4 slides (96 wells) per run:
- Qualitative analysis: up to 96 samples per run
- Quantitative analysis in singlicate: up to 87 samples per run
- Quantitative analysis in triplicate: 28 samples in triplicate per run
Software
- Flexible experimental setup lets you define standard curve and sample placement, and number of dilutions in the standard curve.
- Automated imaging and analysis can be customized using automated user-defined data processing, including normalization, outlier mining, replicate analysis and more.
- 14 embedded curve fits, and the ability to modify protocols to perform a wide variety of mathematical analyses on your data.
- Experimental setup and data analysis can be performed at your desk with standalone VaxArray Software.
- Digital records and automated data analysis are 21 CFR Part 11-compliant.
Relevant Resources