Introducing OmniFlu HA
Simplify Influenza Vaccine Production
OmniFlu HA is an off-the-shelf, validated solution for seasonal influenza vaccine manufacturers’ unique challenges. Engineered for hemagglutinin (HA) quantification and detection, OmniFlu HA features a novel design that provides the specificity and production platform flexibility that next-gen vaccine manufacturers need.
- Convenience — Save time and resources with our ready-to-use kit, eliminating the hassle of sourcing reagents and establishing new protocols.
- Confidence — Every seasonal strain is validated for use on the World Health Organization (WHO)-recommended influenza strains.
- Versatility — A tailored panel of validated capture reagents enables egg-based, cell-based, or recombinant influenza HA analysis from a single kit.

High Specificity, Broad Reactivity
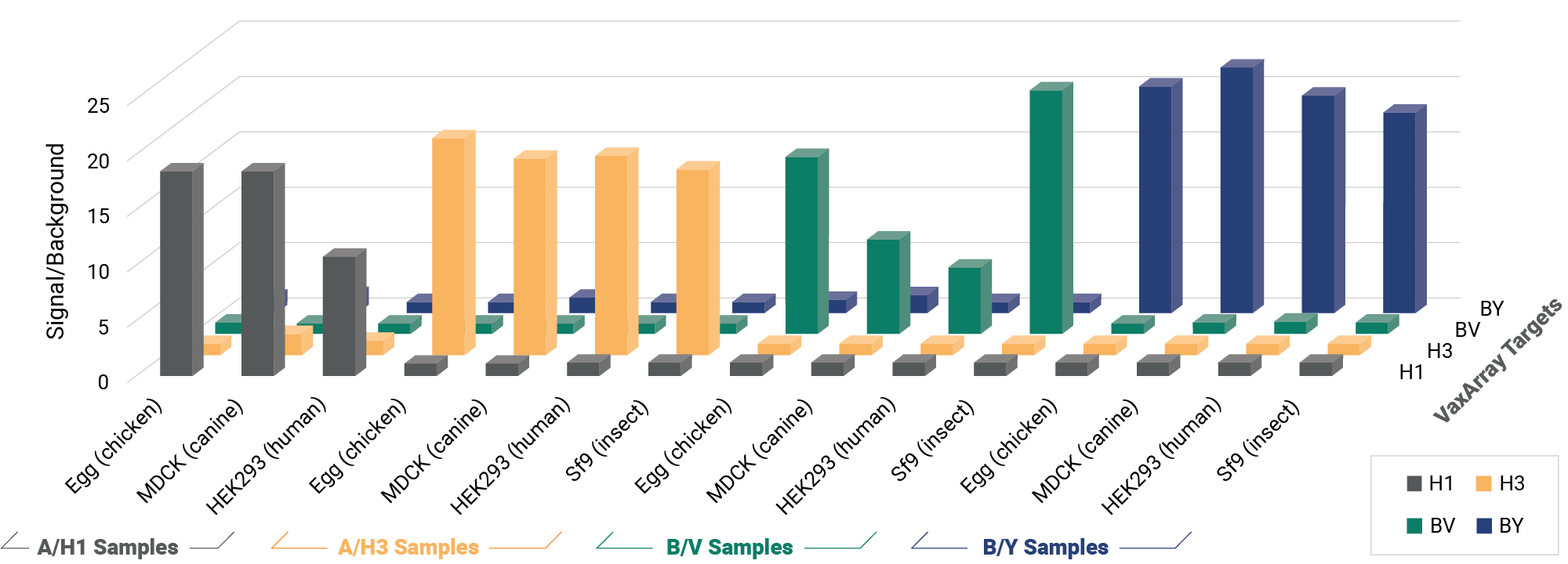
The OmniFlu Hemagglutinin (HA) Assay contains multiple antibodies per influenza subtype for comprehensive multivalent detection of influenza HA from a wide range of expression systems, including egg-based, cell-based, recombinant, and proteins expressed after mRNA-transfection. The capture reagents are pre-validated for high specificity between influenza A/H1, A/H3, B/Victoria and B/Yamagata subtypes and lineages across every WHO biannual strain recommendation.
Product Details
| Description | Specification |
|---|---|
| Format | 16 Chamber Microarray Slide |
| Kit Size | 2 Slides, 32 Samples and/or Standards |
| Compatible Production Platform | Egg-based Cell-based (MDCK) Recombinant (Sf9) mRNA-expressed (HEK-293, Hep3b) |
| Target HAs | H1, H3, B/Victoria, B/Yamagata |
| Seasonal Reactivity Assessment | Reactive with most recent WHO strain recommendations (egg and cell) for Northern and Southern Hemisphere |
| Time to Result | < 2 hours |
| Instrument | VaxArray |
| Software | Version 3.X |
Streamline Your Production
OmniFlu HA is only one of the innovative kits designed for use on the VaxArray® Platform. This easy-to-use benchtop device enables vaccine developers and manufacturers to simplify and standardize their workflows. Explore additional kits and learn how the VaxArray Platform can help you optimize your production line.







