Expert Services
Accelerate Vaccine Development with Customized Solutions
Our Expert Services team provides vaccine manufacturers with tailored workflow solutions to help address and eliminate development bottlenecks, and accelerate analysis and validation.
With over two decades of experience in custom vaccine assay development and harnessing a suite of sophisticated assay development tools and curated reagents, Expert Services combines the reliability and precision of our existing VaxArray® Platform with the flexibility of custom-tailored services. This hybrid approach ensures all clients’ unique requirements are met with the highest standards of accuracy and efficiency.
- Expertly designed, customized assays for your vaccine development and manufacturing save months developing, optimizing, and validating in-house assays
- Multivalent vaccine sample testing in under an hour eliminates days, weeks or months of testing with traditional methods
- Multiplexed assay technology enables quicker analysis over traditional single-plex methods like ELISA
- VaxArray is used as a release assay and accepted by regulatory agencies, including the FDA and Health Canada
- Custom solutions that integrate into your development and manufacturing bioprocesses
Trusted partner of biopharmaceutical companies worldwide for the development of custom, efficient solutions to vaccine development and manufacturing challenges.
“InDevR’s commitment to ensuring that the reagent kits are responsive to seasonal influenza strain changes saved our development team significant time and resources.”
Michael Schrader
CEO of Vaxess
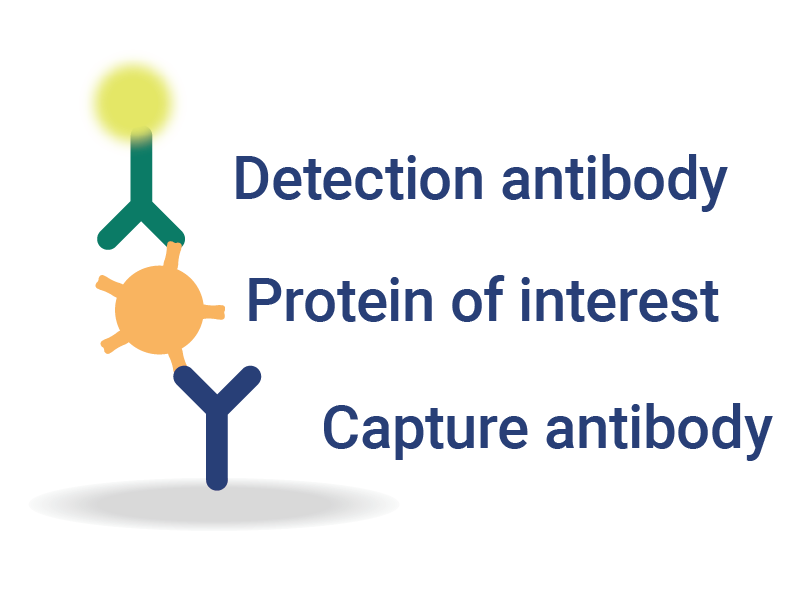
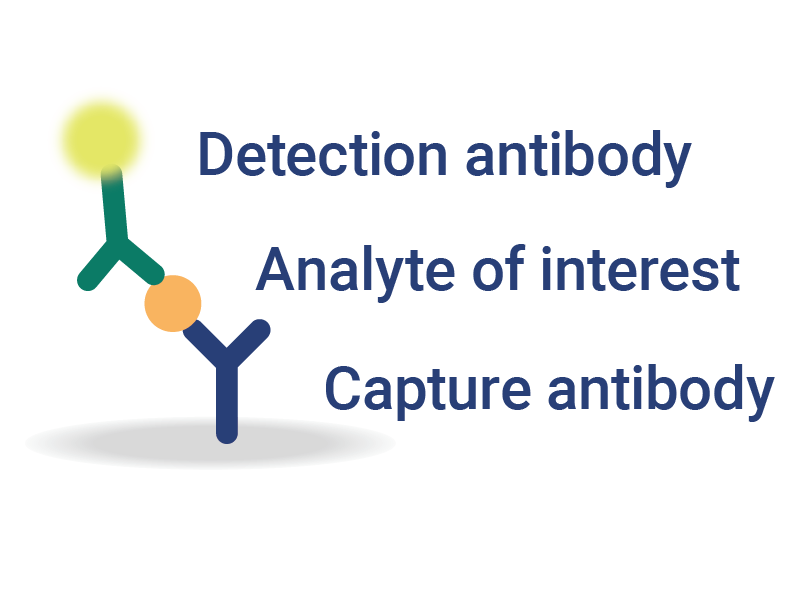
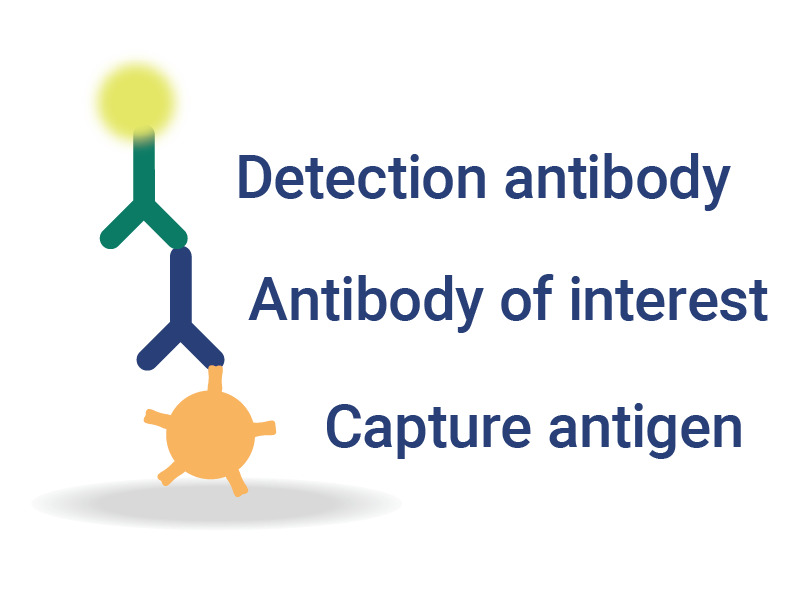
Multiplexed Detection and Quantification
The VaxArray Difference
InDevR’s automated, multiplex assay solutions for vaccine testing consolidate multiple assays into a single test, significantly reducing testing times and improving overall assay sensitivity.
- Flexible platform enables analysis of antibodies, antigens, or nucleic acids
- Curated library of over 300 antibodies enables quick development of multivalent vaccine testing assays to get seasonal influenza vaccines market-ready faster
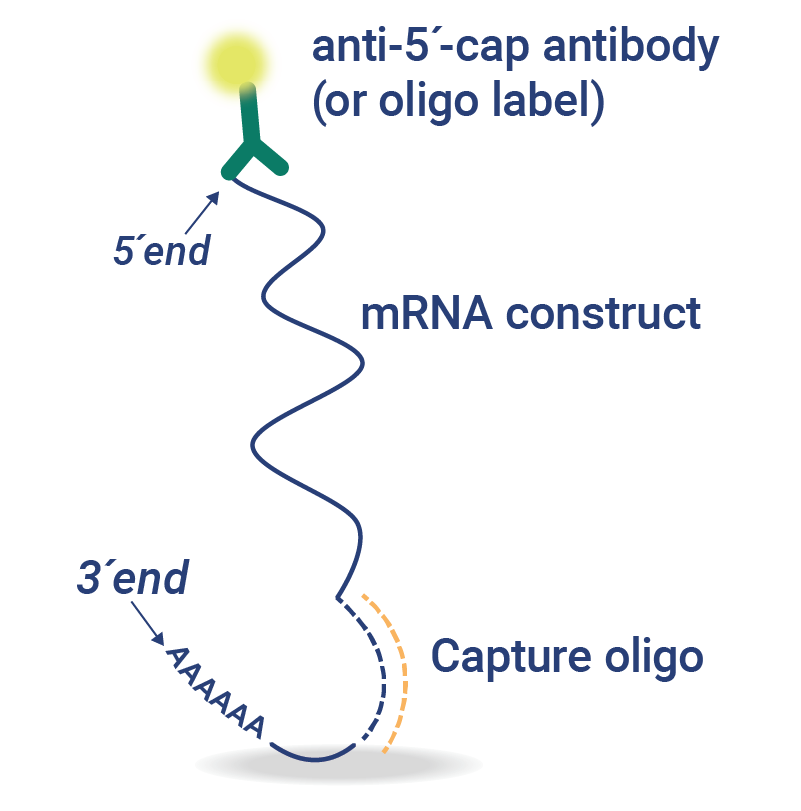
- Specific quantification of LNP-encapsulated mRNA constructs enables analysis without the need for extraction or amplification
- Utilizing multiplex immunoassays enables rapid quantification of cell-expressed protein after mRNA transfection
- Custom assay kits are manufactured under an ISO13485-certified quality system and implemented on a system with 21 CFR Part 11-compatible software
- Flexible platform enables analysis of antibodies, antigens, or nucleic acids
- Curated library of over 300 antibodies enables quick development of multivalent vaccine testing assays to get seasonal influenza vaccines market-ready faster
- Specific quantification of LNP-encapsulated mRNA constructs enables analysis without the need for extraction or amplification
- Utilizing multiplex immunoassays enables rapid quantification of cell-expressed protein after mRNA transfection
- Custom assay kits are manufactured under an ISO13485-certified quality system and implemented on a system with 21 CFR Part 11-compatible software
Our Development Process*
1
Feasibility
~1 month
Quick assessment of project feasibility to establish proof of concept for actionable information.
2
Prototype Kits
~1 week
Ability for current VaxArray users to evaluate custom kit in-house
3
Optimization & Performance Assessment
~2 months
Microarray & assay optimization followed by assessment of assay performance with customer-designated samples
4
Manufacturer Transfer
~1 month
QC test verification & documentation, manufacturing verification & document
*Timeline is estimated based on the average time of 35 projects; exact timelines are project dependent.
Example Applications
Seasonal Influenza Vaccine and Biosurveillance
- Hemagglutinin-coding mRNA construct identification and quantification: Assays designed for rapid, multiplexed identification and quantification of LNP-encapsulated quadrivalent mRNA (coding for hemagglutinin) vaccine drug substances without the need for labor-intensive mRNA extraction steps.
- Neuraminidase protein identification and quantification: Assays for specific identification and sensitive quantification of multiple neuraminidase subtypes in mixtures of recombinant proteins, including stability assessment to inform the vaccine development processes.
- Host cell protein quantification: VaxArray can replace home-brew ELISA, often plagued with poor reproducibility.
- Protein identification and quantification following mRNA transfection: Examine expressed influenza proteins after transfection of a multivalent HA and NA-containing influenza mRNA drug product as a function of input mRNA dose or other transfection variables.
- Quantification of serum antibodies: Preclinical and clinical support through multiplexed evaluation of antibody response in serum following immunization with multivalent mRNA vaccine designed to induce multiple hemagglutinin and neuraminidase proteins.
- Hemagglutinin-coding mRNA construct identification and quantification: Assays designed for rapid, multiplexed identification and quantification of LNP-encapsulated quadrivalent mRNA (coding for hemagglutinin) vaccine drug substances without the need for labor-intensive mRNA extraction steps.
- Neuraminidase protein identification and quantification: Assays for specific identification and sensitive quantification of multiple neuraminidase subtypes in mixtures of recombinant proteins, including stability assessment to inform the vaccine development processes.
- Host cell protein quantification: VaxArray can replace home-brew ELISA, often plagued with poor reproducibility.
- Protein identification and quantification following mRNA transfection: Examine expressed influenza proteins after transfection of a multivalent HA and NA-containing influenza mRNA drug product as a function of input mRNA dose or other transfection variables.
- Quantification of serum antibodies: Preclinical and clinical support through multiplexed evaluation of antibody response in serum following immunization with multivalent mRNA vaccine designed to induce multiple hemagglutinin and neuraminidase proteins.
SARS-CoV-2 and Other Coronavirus Vaccine and Biosurveillance
- Bivalent mRNA quantification: Demonstrate specificity and sensitivity for multiple mRNA constructs coding for SARS-CoV-2 spike protein variants.
- Spike protein quantification: Detection and quantification of S1 and S2 subunits of SARS-CoV-2 spike vaccine drug products.
- Reactivity of serum antibodies: Evaluation of induced cross-protection for VLP-based spike protein Drug Product by multiplexed analysis of serum antibodies against both wild-type and variant antigens.
- Quantification of serum antibodies: Multiplexed quantification of antibodies against SARS-CoV-2 and endemic CoV in human serum to support clinical trials.
- Bivalent mRNA quantification: Demonstrate specificity and sensitivity for multiple mRNA constructs coding for SARS-CoV-2 spike protein variants.
- Spike protein quantification: Detection and quantification of S1 and S2 subunits of SARS-CoV-2 spike vaccine drug products.
- Reactivity of serum antibodies: Evaluation of induced cross-protection for VLP-based spike protein Drug Product by multiplexed analysis of serum antibodies against both wild-type and variant antigens.
- Quantification of serum antibodies: Multiplexed quantification of antibodies against SARS-CoV-2 and endemic CoV in human serum to support clinical trials.
Pneumococcal Conjugate Vaccine and Biosurveillance
- Polysaccharide Antigen Quantification: Multiplexed quantification of polysaccharides, both native and protein conjugated, in pneumococcal conjugate vaccine drug products to significantly reduce time to result compared to traditional singleplex light scattering assays.
- Quantification of Serum Antibodies in Support of Preclinical Studies: Comparative assessments of serum antibody levels induced by highly multivalent pneumococcal conjugate vaccines delivered by novel microneedle patches relative to traditional needles to support device development efforts.
- Polysaccharide Antigen Quantification: Multiplexed quantification of polysaccharides, both native and protein conjugated, in pneumococcal conjugate vaccine drug products to significantly reduce time to result compared to traditional singleplex light scattering assays.
- Quantification of Serum Antibodies in Support of Preclinical Studies: Comparative assessments of serum antibody levels induced by highly multivalent pneumococcal conjugate vaccines delivered by novel microneedle patches relative to traditional needles to support device development efforts.
Publications
Byrne-Nash, R.T., et al. VaxArray potency assay for rapid assessment of “pandemic” influenza vaccines. 2018. NPJ Vaccines. 3:43; doi:10.1038/s41541-018-0080-6
Kuck L.R., et al. VaxArray assessment of influenza split vaccine potency and stability. 2017. Vaccine. 15(35) 1918-1925. doi:10.1016/j.vaccine.2017.02.028
Hu T., et al. 30-Minute highly multiplexed VaxArray immunoassay for Pneumococcal vaccine antigen. 2022. Vaccines. 10(11): 1964. doi: 10.3390/vaccines10111964
Gao R.Y., et al. Rapid identity and auantity CQA test for multivalent mRNA drug product formulations. 2022. Vaccines. 10(10): 1704. doi: 10.3390/ vaccines10101704
Dawson, E.D., et al. VaxArray immunoassay for the multiplexed quantification of poliovirus D-antigen. 2022. J Immunol Methods. 504:113259. doi: 10.1016/j.jim.2022.113259
Gillis J.H., et al. Multiplexed VaxArray immunoassay for rapid antigen quantification in measles and rubella vaccine manufacturing. 2021. Vaccine: X. 9:100113. doi: 10.1016/j.jvacx.2021.100113